What Can Be Used to Represent Bond Dipole Moments?
What is Dipole Moment?
A dipole moment arises in any system in which there is a separation of charge. They tin, therefore, arise in ionic bonds besides as in covalent bonds. Dipole moments occur due to the difference in electronegativity between ii chemically bonded atoms.
A bond dipole moment is a measure of the polarity of a chemic bond between 2 atoms in a molecule. It involves the concept of electric dipole moment, which is a measure of the separation of negative and positive charges in a system.
The bail dipole moment is a vector quantity since it has both magnitude and direction. An illustration describing the dipole moment that arises in an HCl (hydrochloric acrid) molecule is provided below.

It tin be noted that the symbols 𝛿+ and 𝛿– stand for the two electric charges that arise in a molecule which are equal in magnitude simply are of opposite signs. They are separated by a set distance, which is commonly denoted by 'd'.
Important Points
- The dipole moment of a single bond in a polyatomic molecule is known equally the bail dipole moment and it is dissimilar from the dipole moment of the molecule as a whole.
- It is a vector quantity, i.e. it has magnitude as well as definite directions.
- Existence a vector quantity, it tin can also be zilch as the two oppositely interim bail dipoles can cancel each other.
- By convention, information technology is denoted by a modest arrow with its tail on the negative center and its caput on the positive center.
- In chemistry, the dipole moment is represented past a slight variation of the arrow symbol. It is denoted past a cross on the positive center and arrowhead on the negative center. This arrow symbolizes the shift of electron density in the molecule.
- In the case of a polyatomic molecule, the dipole moment of the molecule is the vector sum of the all nowadays bond dipoles in the molecule.
Recommended Videos

Dipole Moment Formula
A dipole moment is the product of the magnitude of the charge and the altitude between the centers of the positive and negative charges. It is denoted past the Greek letter 'µ'.
Mathematically,
Dipole Moment (µ) = Accuse (Q) * distance of separation (r)
It is measured in Debye units denoted by 'D'. 1 D = iii.33564 × 10-30 C.k, where C is Coulomb and 1000 denotes a meter.
The bond dipole moment that arises in a chemic bond between 2 atoms of different electronegativities can be expressed equally follows:
μ = 𝛿.d
Where: μ is the bond dipole moment,
𝛿 is the magnitude of the partial charges 𝛿+ and 𝛿–,
And d is the distance between 𝛿+ and 𝛿–.
The bond dipole moment (μ) is also a vector quantity, whose direction is parallel to the bond axis. In chemistry, the arrows that are drawn in society to represent dipole moments brainstorm at the positive charge and end at the negative charge.
When two atoms of varying electronegativities interact, the electrons tend to move from their initial positions to come up closer to the more than electronegative atom. This movement of electrons can be represented via the bond dipole moment.
Examples
Dipole moment of BeFtwo
In a beryllium fluoride molecule, the bond angle between the two beryllium-fluorine bonds is 180o. Fluorine, existence the more electronegative atom, shifts the electron density towards itself. The individual bond dipole moments in a BeFtwo molecule are illustrated below.

From the illustration provided in a higher place, it can be understood that the two individual bond dipole moments abolish each other out in a BeFii molecule because they are equal in magnitude just are contrary in direction. Therefore, the net dipole moment of a BeF2 molecule is zero.
Dipole moment of H2O (Water)
In a water molecule, the electrons are localized effectually the oxygen atom since it is much more electronegative than the hydrogen atom. However, the presence of a lone pair of electrons in the oxygen atom causes the water molecule to take a bent shape (as per the VSEPR theory). Therefore, the individual bond dipole moments do non cancel each other out as is the instance in the BeFtwo molecule. An illustration describing the dipole moment in a h2o molecule is provided below.
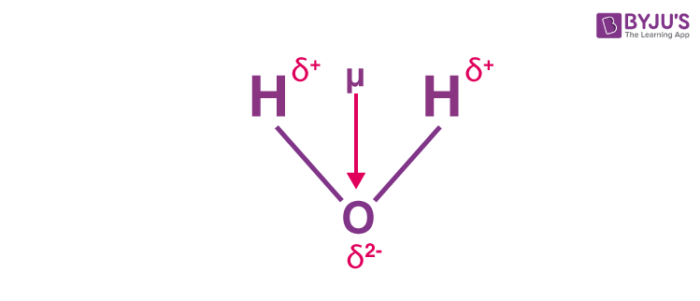
The bail angle in a water molecule is 104.5o. The individual bond moment of an oxygen-hydrogen bond is one.v D. The net dipole moment in a water molecule is found to exist 1.84D.
Frequently Asked Questions – FAQs
Why is it called a dipole moment?
If an object is balanced, the theory of moments is that the sum of the clockwise moments around a pivot is equal to the sum of the anticlockwise moments around the same pivot. To explain this, I assumed it was chosen a dipole moment, since it defines the molecule'due south range of motion.
What is meant past dipole moment and dipole moment?
An electrical dipole is considered an arrangement of two equal and reverse charges divided by a finite altitude. An electric dipole's electric dipole moment is defined as the result of each of its charges and the dipole's length. It is the sum of a vector and is defined past p.
How practice y'all notice the largest dipole moment?
When there is a difference in the electronegativity of two atoms involved in a bail, a dipole moment happens. The larger the electronegativity difference between the two atoms, the larger the bail'due south dipole moment and polarity.
How practice you lot find the dipole moment of co2?
Carbon dioxide has a linear geometry at the core with carbon and oxygen on both sides while oxygen is more electronegative than carbon electron deject is pushed to oxygen and both oxygen pull the electron deject from both sides with the aforementioned tendency, so the internet effect is zero.
What is the symbol of dipole moment?
The dipole moment (μ) is the adding of the internet molecular polarity at either terminate of the molecular dipole, which is the magnitude of the charge Q times the altitude r between the charges. Dipolar moments tell u.s.a. of the division of charges in a molecule.
Thus, the definition and formula of dipole moments are briefly discussed in this article. To learn more about this concept and other related concepts, such as polarity, register with BYJU'Southward and download the mobile awarding on your smartphone.
whiteheadprucestras.blogspot.com
Source: https://byjus.com/chemistry/dipole-moment/
0 Response to "What Can Be Used to Represent Bond Dipole Moments?"
Publicar un comentario